Torrent Pharmaceuticals Limited on Tuesday expanded a recall of 10 lots of Losartan potassium tablets to include six lots of Losartan potassium and hydrochlorothiazide tablets due to the detection of a potential cancer-causing impurity. It's the latest in more than two dozen such alerts for blood pressure medications from various companies since July 2018 and the second in four days.
The impurity is called N-nitrosodiethylamine (NDEA). It is classified as a probable human carcinogen by the International Agency for Research on Cancer. The National Institutes of Health website said NDEA is used as gasoline and lubricant additive, antioxidant and a stabilizer for industry materials.
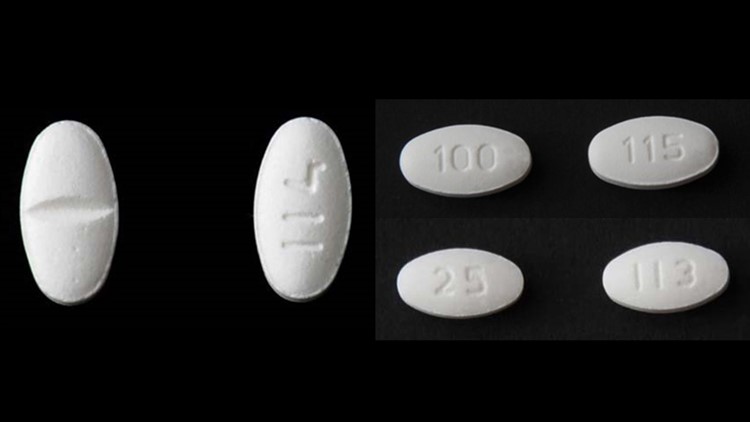
Here is a full list from Torrent of the recalled Losartan products.
The company said in Tuesday's alert that there have been no reports of "adverse events" related to the recall.
Losartan is used to treat hypertension, hypertensive patients with Left Ventricular Hypertrophy and for the treatment of nephropathy in Type 2 diabetic patients, the company said.
Despite the possible cancer risk, the company said patients who are using Losartan should continue taking them. That's because the health risk to patients could be higher if they stop without an alternate form of treatment. Patients are advised to contact their doctors about alternatives.
You can use this link to search for recent drug recalls
The FDA told USA TODAY late last year it was investigating the root cause of the problem that is leading to all the recalls.