It all comes down to this.
A vote by an FDA panel will determine if the coronavirus vaccine will be approved for emergency use and if millions of doses will start shipping around the United States.
The meeting started at 9 a.m. ET and consisted of a discussion by panelists including various independent epidemiologists and infectious disease experts.
There will be robust discussion throughout the day before a vote is expected late in the afternoon Thursday.
The vote comes down to one singular question:
Based on the totality of scientific evidence available, do the benefits of the Pfizer vaccine outweigh its risks for use in individuals 16 years of age and older?
A doctor with the U.S. Food and Drug Administration began by introducing how we got to this stage.
Pfizer submitted an Emergency Use Authorization request by the FDA on November 20. The company is asking that it be approved for people over the age of 16. Along with the request, Pfizer submitted safety and efficacy data from their randomized, blind, placebo-controlled Phase 3 trial.
Immediately following the introduction, doctors started asking questions. One panelist wanted to know if they had to approve the vaccine for Pfizer's age requirement or could they elect to go with 18 years and older. Another asked what impact this EUA will have on other vaccine trials in the future.
Another doctor noted that Pfizer's submitted data only went through mid-November and requested more current data.
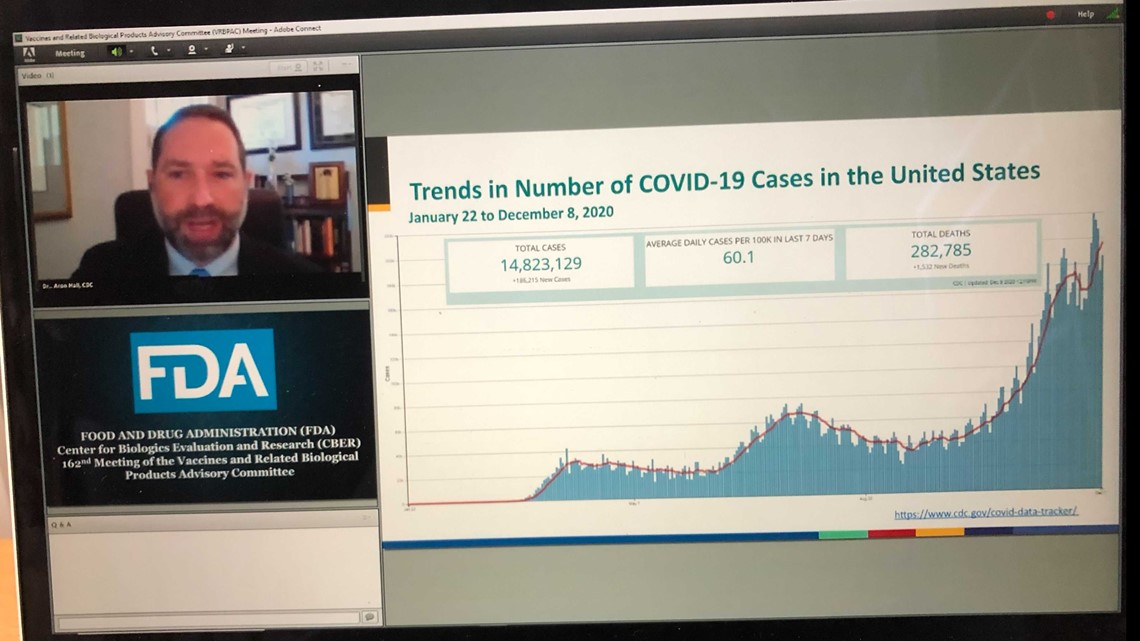
After an initial discussion about the vaccine, the meeting took a turn to focus on the state of the pandemic in the U.S. with an update from Dr. Aaron Hall, a member of the CDC's COVID-19 task force.
Hall explained there have been roughly 15 million COVID-19 cases in the U.S. but estimates the number could be two to seven times greater than that.
Deaths had reached 282,785 by December 8.

After a briefing on the coronavirus, another doctor with the FDA summarized the complications that will follow EUA regarding the integrity of all COVID-19 vaccine trials already underway.
The panel took a short break at 11:45 am. They returned to hear from doctors and professors from around the country calling in to offer their opinions in an open public hearing. One of the callers was a Tampa pediatrician, who urged the committee to build vaccine confidence to ensure informed consent among those receiving it.
The open hearing is scheduled to last one hour before Pfizer representatives present data.
- Fired COVID-19 data worker accused of accessing state system says Florida made the login info public
- Tampa boil water notice rescinded
- How to watch the FDA's Pfizer vaccine meeting
- Panel gives Pfizer COVID-19 vaccine final look Thursday before US decision
- Here's where to see Christmas lights around Tampa Bay
►Breaking news and weather alerts: Get the free 10 Tampa Bay app
►Stay In the Know! Sign up now for the Brightside Blend Newsletter

