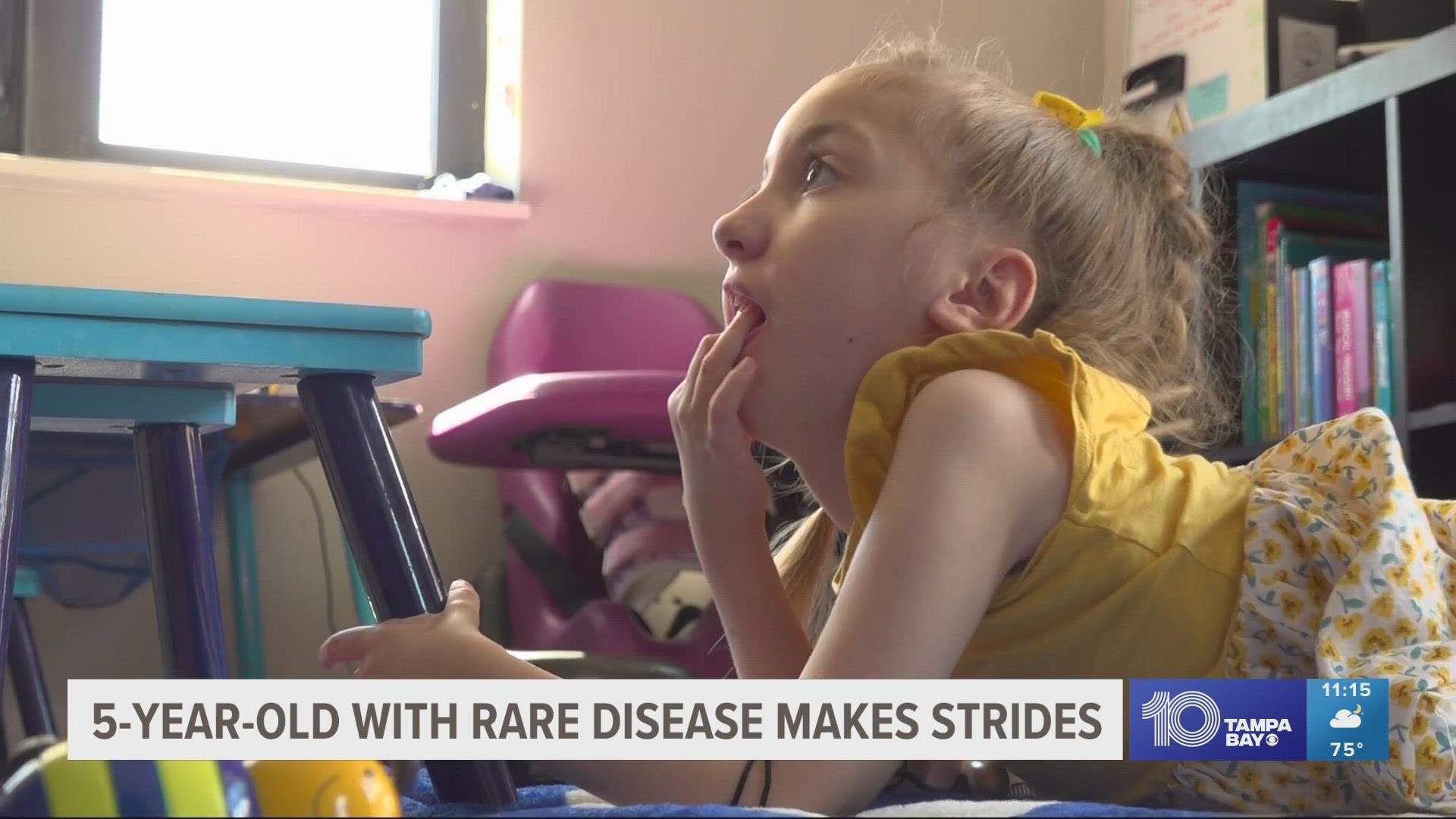
ST. PETERSBURG, Fla. — Emma Schnyders is young, but she beats the odds every single day.
"Emma is just…pure joy," her father Ryan said.
Ryan says, when she was about 6 months old, they noticed she wasn’t hitting developmental milestones.
"Emma had stopped and lost function in her arms and legs," Ryan said.
He says she was diagnosed with Type 1 Spinal Muscular Atrophy (SMA).
It's a disease that experts say can cause muscle weakness, reduced movement, and problems with swallowing and feeding. Doctors said, now 5-year-old Emma, only had a life expectancy of age two.
"Going from a terminal diagnosis to having a daughter who can roll around and function and respond to you,” Ryan said. “She'll say ‘Mama’ and ‘Dada’ and at first we were told that would never happen. We were told she would never sit up unassisted."
Ryan says Emma has made big strides with a new treatment called Evrysdi.
It's the first FDA-approved at-home oral treatment for SMA, and Dad says, it's been life-changing. Prior treatments…were a challenge.
"The day in the hospital, the two days of recovery, the headaches, and everything else that comes with it,” he said “We don't have to worry about that anymore."
Ryan says each day offers a new opportunity for Emma to show us just what she'll do next.
"Trust God, trust the process because he's got a bigger plan for us than we know,” he said. “Emma has been an example of that from birth. Anytime they set a limitation, she crushes it."
Studies show that SMA is the leading genetic cause of infant death.
Checking for SMA in newborns has been added to the national recommended list of disorders that babies are screened for at birth.
The FDA has expanded approval of Evrysdi for babies under 2 months old, so children are able to get the treatment as early as possible.